Special Summer Series: Elizabeth Hoover de Galvez from the library's reference department, shares her observations of summer research at Coe. This summer she is working with Dr. Feller's materials science group.
For all of Dr. Feller's materials science students, the first week's activity was to make a sample of glass (.4 Sodium Borate) and then run three tests on it (Raman spectrography to get the molecular structure, DSC to get the glass transition temperature, or Tg, which is the point at which the glass begins to soften, and Pycnometer for a density measurement). Even if the first sample turns out perfectly, Dr. Feller says that students should then make the sample a second time and then a third. My lab partner, who has a lot of experience in the lab rolls her eyes; she has apparently made this glass too many times before and wants to get back to making a more useful sample. But for me it sounds like a great opportunity to see what making glass is all about and then to get comfortable with the procedures.
The first day, I watch my lab partner go through the steps, which gives me plenty of opportunity to take copious notes. Dr. Feller stresses the importance of a thorough lab notebook. He wants the story of the experiments recorded, including all of the procedures, errors, and unexpected results. He warns his students that if they don't write it all down, then when it comes time to write a paper for publication or to replicate the experiment, perhaps months or years later, everyone will be lost.
While making and testing the first sample, I am surprised by how comfortable I feel with many aspects of the procedure. We start out using Google Sheets to calculate the recipe for our glass; since I love tinkering with Excel, (and because my lab partner knows exactly what she's doing), I don't feel at all as intimidated by this first step as I thought I would. When it comes time to actually measure out the ingredients on the scale, the process is familiar from my college chemistry labs. While the 1000 degree C (1800 degrees F) glowing orange furnaces are enough to scare anyone, I am relieved that there are at least no open flames. And when we open up the Raman spectroscopy machine to put
our sample in the first time, I am delighted to see a microscope, which is one piece of lab equipment I got very comfortable with in my college biology lab classes. The sample of glass under 10x magnification even looks like bone tissue to me, a gorgeous network of transparent blue struts, similar to the photo at left. Rather than feeling completely alienated, I feel fairly at home in this high-tech, multi-million dollar physics lab.
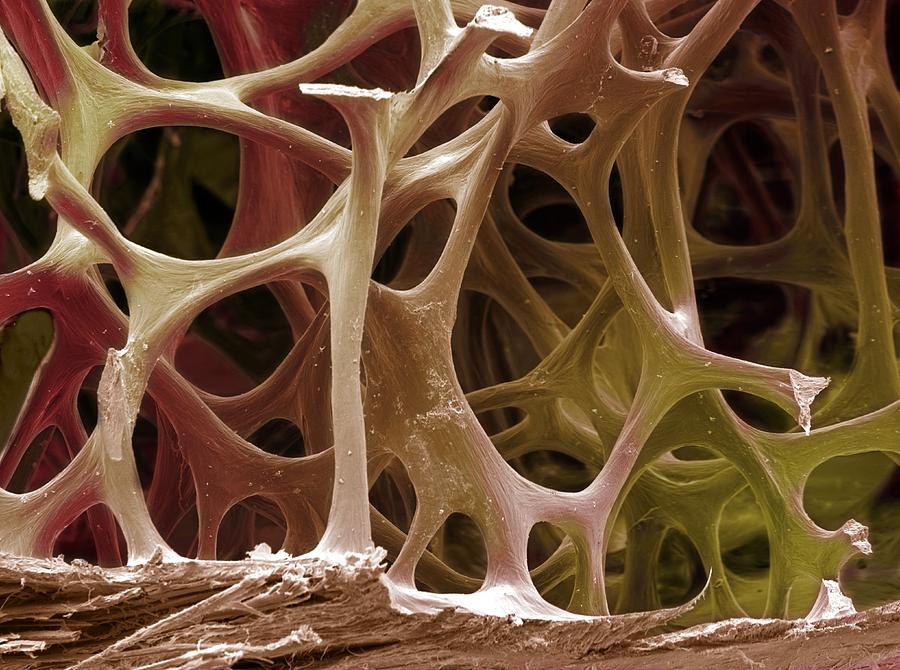 |
Bird bone tissue. Coloured scanning electron
micrograph (SEM) of spongy bone from a robin.
By Steve Gschmeissner. |
In addition to some familiar equipment, all of the people in the lab--both students and Doctor Feller--put me at ease. They are extremely welcoming, excited to be working there, and happy to share their passions with a novice. Doctor Feller remembers to point out the physics concepts in basic terms for me and I think I can remember some of the concepts from my introductory level science classes.
All in all, the first day nerves get squelched very quickly and I immediately start learning much faster than I would from a book or article. On my way out on my first day, I pass a student poster hanging in the hall and stop to read it. Rather than being confused by the terminology and descriptions of the procedure, I quickly grasp the basics.
No comments:
Post a Comment